5 May 2017
Belleville, Ontario – NovaVive Inc., an animal health immunobiology company, is pleased to announce that it is launching an equine-specific version of its Immunocidin® product for equine sarcoid tumors in response to demand by U.S. veterinarians. The new product, Immunocidin® Equine, is packaged in 5mL vials for the convenience of practitioners. The Company believes that there is no other regulator-approved equine sarcoid therapy in North America.
Equine sarcoids are locally aggressive fibroblastic neoplasms considered to be the most common skin tumors of horses worldwide. They are often found around the eyes, head/face, neck, chest, and shoulder, and at the site of old scars. Young to middle-aged horses are most commonly affected by sarcoids, and it is estimated that sarcoids affect 1 in 100 horses in North America.
Current treatment options include surgery, ligation, cryotherapy, topical treatment, chemotherapy, radiation therapy, or laser removal; many of these treatments incur side effects. Immunotherapy is a safe and effective treatment option.
Immunocidin® Equine is administered by intratumoral injection, but the response is generalized and untreated sites frequently undergo regression as well.
“Immunocidin Equine has a high post-treatment tumor-free rate and is well-tolerated by horses, including older animals,” said Dr. Stan Alkemade, Chief Veterinary Scientific Officer at NovaVive Inc. “The product has minimal side effects and an excellent safety profile. It offers an economical treatment option to veterinarians.”
Here are before and after pictures of a horse treated with Immunocidin. The second picture was taken two weeks after four injections (administered at 14-day intervals).
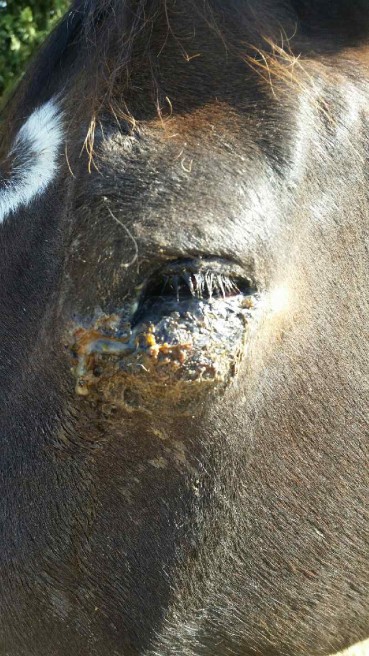

About NovaVive Inc.
NovaVive is a private company founded in July, 2014. The Company has an advanced veterinary immunotherapeutic platform based on mycobacterium cell wall fraction technology with 5 regulator-approved products. Some of these products have demonstrated the capability of reducing the reliance on antibiotics in the treatment of certain diseases of horses and cattle. The Company’s development plan is to identify additional livestock and companion animal diseases that may be effectively treated with its immunotherapeutic technology platform. For more information about Immunocidin Equine or the Company’s other products, visit www.NovaVive.ca.
For further information, contact:
Graeme McRae, President
Graeme.McRae@NovaVive.ca
Phone: 613-391-3837
or
Jennifer Shea, Vice-President, Investor Relations & Business Development
Jennifer.Shea@NovaVive.ca
Phone: 613-391-2097